You’ve probably seen old aluminum siding turn dull gray. Or noticed white powder forming on an aluminum ladder. Your first thought might be: “Is that rust?”
Here’s the thing that confused me when I first studied chemistry: we use “rust” and “corrode” like they mean the same thing. They don’t.
The short answer? Aluminum can’t rust. But it definitely corrodes.
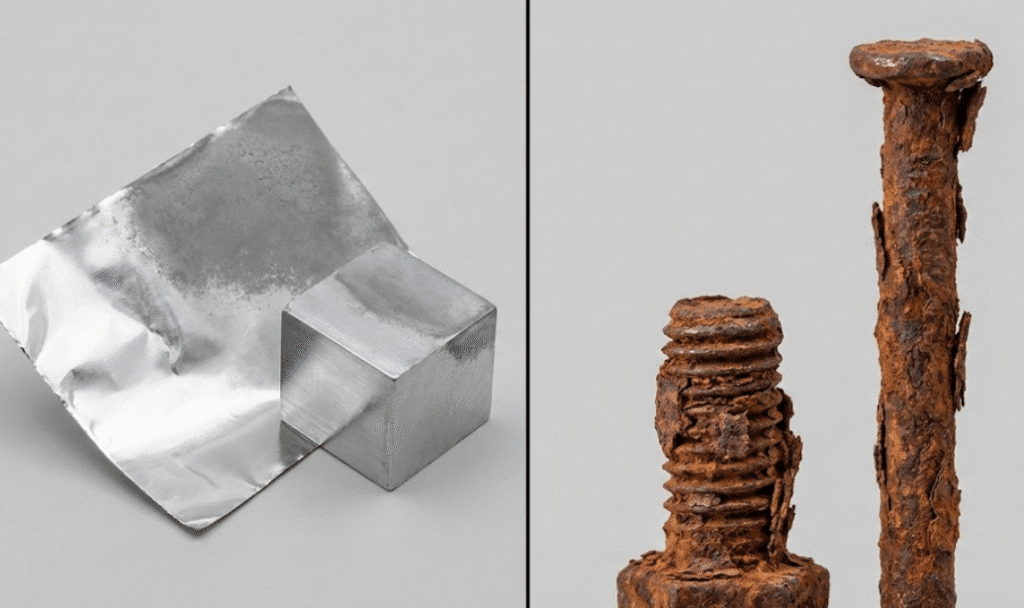
These two terms trip up even smart people. Let’s break them down.
Rust is iron oxide—specifically Fe₂O₃. It only forms on iron and metals that contain iron, like steel.
Think about that rusty nail in your garage. The reddish-brown flakes you see? That’s iron atoms bonding with oxygen and water. The chemical reaction looks like this: 4Fe + 3O₂ + 6H₂O → 4Fe(OH)₃.
Here’s what makes rust so destructive: it’s flaky and porous. Water and oxygen keep seeping through, attacking more metal underneath. A rusted nail doesn’t just look bad—it’s literally crumbling away.
Corrosion is the broader term. It means any metal breaking down through chemical reactions with its environment.
Rust is just one type of corrosion—the type that happens to iron. Copper turns green. Silver tarnishes black. Aluminum forms a white-gray coating. All corrosion, but none of it is rust.
Here’s the key insight: all rust is corrosion, but not all corrosion is rust. It’s like squares and rectangles—every square is a rectangle, but not every rectangle is a square.
| Term | What It Means | What Metals It Affects |
|---|---|---|
| Corrosion | Any metal degrading through chemical reaction | All metals |
| Rust | Specifically iron oxide formation | Only iron and steel |
No. Aluminum cannot rust. Period.
Rust requires iron atoms. Aluminum has zero iron in it. No iron means no rust—it’s chemically impossible.
This isn’t my opinion. It’s basic chemistry. You could leave a piece of pure aluminum underwater for a hundred years, and it would never produce a single speck of rust.
I get why people think aluminum rusts. When you see that dull gray coating on old aluminum, it looks like something’s wrong.
But that gray appearance isn’t damage. It’s actually protection. The white or gray powder on aged aluminum is aluminum oxide—and it works completely differently than rust.
People also use “rust” casually to mean any metal degradation. When your neighbor says their aluminum gutter is “rusting,” they probably mean it’s corroding. The words get mixed up in everyday speech.
Here’s where aluminum gets interesting. It reacts with oxygen faster than almost any other metal—but that reaction saves it instead of destroying it.
The moment you cut a piece of aluminum, it starts reacting with oxygen. This happens within 100 picoseconds—that’s 0.0000000001 seconds.
The chemical reaction is simple: 4Al + 3O₂ → 2Al₂O₃.
Scientists call this “passivation.” The aluminum isn’t being damaged. It’s building armor.
A thin layer of aluminum oxide (Al₂O₃) covers the entire surface. This layer is incredibly thin—only 2 to 5 nanometers thick. That’s about 50,000 times thinner than a human hair.
But here’s what amazes me: despite being nearly invisible, this layer forms a complete, airtight seal over the metal. Oxygen can’t get through to attack the aluminum underneath.
Unlike iron, aluminum’s oxidation is self-limiting. Once that thin oxide layer forms, the reaction stops.
The oxide layer blocks any more oxygen from reaching the fresh metal. Result? Your aluminum is protected, not destroyed.
Fresh aluminum looks shiny and silver. Give it a few hours exposed to air, and it turns dull gray. That color change means the protection is working.
This is my favorite part of aluminum chemistry. Two metals oxidize in similar ways, but the results couldn’t be more different.
The difference comes down to structure:
| Property | Aluminum Oxide | Iron Oxide (Rust) |
|---|---|---|
| Structure | Dense and non-porous | Flaky and porous |
| Adhesion | Bonds tightly to metal | Flakes off easily |
| What happens next | Stops further oxidation | Exposes more metal to attack |
| Appearance | Dull gray or white | Red-brown flakes |
| Self-healing | Yes | No |
Iron oxide is porous. Water and oxygen seep right through it to attack the iron underneath. The rust layer keeps growing thicker, and the metal keeps getting weaker.
Aluminum oxide is dense. It seals the surface completely. Nothing gets through.
Scratch a piece of aluminum. You’ve just exposed fresh metal to the air.
What happens? A new oxide layer forms in milliseconds. The scratch heals itself before you can blink.
Scratch iron, and you’ve created a starting point for rust that will spread. Scratch aluminum, and it fixes itself.
Yes. Aluminum isn’t invincible.
The protective oxide layer works great in normal conditions. But certain environments can break through it:
When aluminum does corrode, it usually takes one of these forms:
Let me leave you with the important points:
Aluminum does NOT rust. Rust is specifically iron oxide. No iron, no rust. This is chemistry, not opinion.
Aluminum DOES oxidize. But unlike iron, this oxidation protects the metal instead of destroying it.
The oxide layer is remarkably thin but effective. At just 2-5 nanometers thick, it completely seals the surface and even heals itself when scratched.
Corrosion can still happen. Salt, other metals, and extreme pH environments break through the protection. But these conditions are avoidable.
Next time you see dull gray aluminum, you’ll know what you’re really looking at: protection in action, not damage. That boring gray coating is the reason aluminum ladders don’t crumble like rusted steel nails.

